The projected costs to Medicare from the newly approved Alzheimer’s drug Aduhelm (aducanumab) are astronomical.
“Plenty of other drugs cost more than Aduhelm, which is made by Biogen and will be priced at $56,000 annually,” The New York Times noted on Tuesday. “What makes it different is that there are millions of potential customers, and the drug is expected to be taken for years.”
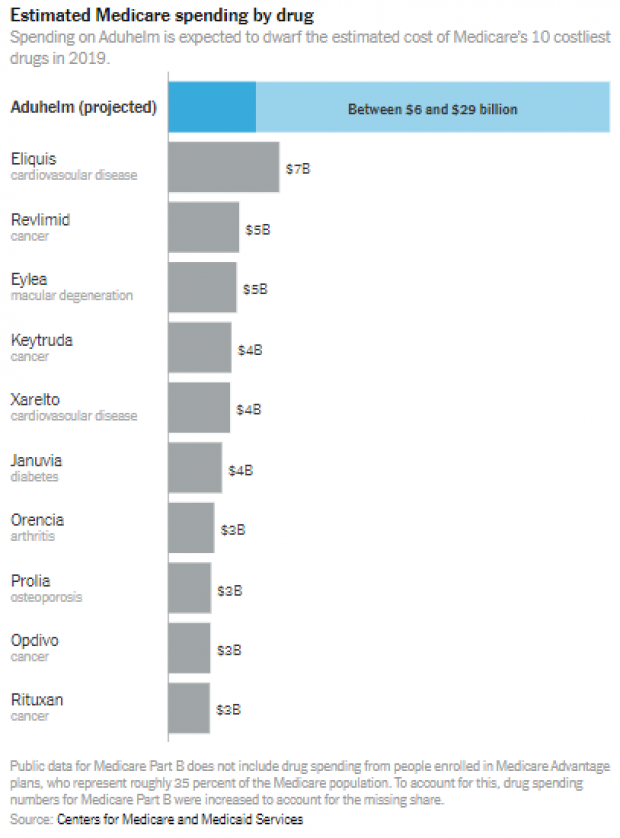
Nearly 6 million Americans have been diagnosed with Alzheimer’s. Despite little evidence that Aduhelm slows the progression of dementia, if 1 million Medicare beneficiaries take the drug, which is administered by intravenous infusion, the cost to Medicare could potentially top $57 billion a year, according to estimates from the Kaiser Family Foundation — $20 billion more than Medicare Part B, which covers physician-administered medications, spent on all drugs combined in 2019. More conservative projections put the annual cost of Aduhelm between $5.8 billion and $29 billion, with the latter number still dwarfing what Medicare spends on any other single drug.
“It’s unfathomable,” said Tricia Neuman of the Kaiser Family Foundation told the Times. “These are crazy numbers.”
The spending on Aduhelm would be borne by taxpayers and by all Medicare beneficiaries, who would face higher premiums. “And the costs are likely to spill over into state budgets, where Medicaid pays premiums for low-income Medicare enrollees,” the Times says.
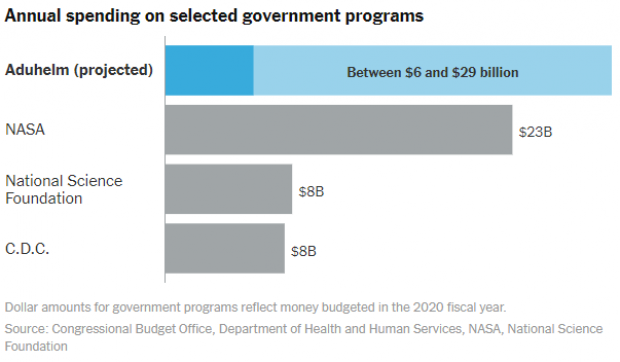
The Times on Tuesday put those numbers in some useful context with a couple of charts showing how the expected costs of Aduhelm compare to Medicare’s 10 costliest drugs in 2019 — and to government programs like NASA and the Centers for Disease Control and Prevention.
Cost-control options: Medicare does have some options for controlling its costs. “It could decide to cover the drug in a way that is more limited than the F.D.A. approval, a break from its normal practice,” the Times notes. Or it could evaluate the drug’s efficacy by putting it into an experiment, covering costs in some regions but not others. “Such policy experiments were authorized under the Affordable Care Act, but one has never been used to limit coverage of a drug in this way,” the Times says.
Sen. Ron Wyden on Tuesday outlined a proposal to lower prescription drug costs, including by allowing Medicare to negotiate drug prices, a Democratic priority opposed by Republicans.