Heather Bresch, the embattled CEO of Mylan pharmaceutical company, expressed modest regret Wednesday afternoon to House lawmakers for price gouging on the highly popular EpiPen epinephrine auto-injector that is used to treat life-threatening allergic reactions.
“Looking back, I wish we had better anticipated the magnitude and acceleration of the rising financial issues for a growing minority of patients who may have ended up paying the [list price] or more,” Bresch said in written testimony. “We never intended this.”
Related: Will Mylan’s $300 Generic EpiPen Packs Quell Pricing Firestorm?
Bresch has been back-peddling for weeks following complaints from Democratic presidential nominee Hillary Clinton, lawmakers and consumer groups about a marketing strategy that drove up the list price of the two-pack nearly six-fold since 2007, from $100 to $600.
EpiPen injectors generate a reported $1 billion a year in revenues for Mylan.
Mylan is just the most recent pharmaceutical company to spark a nationwide uproar over excessive drug pricing. These companies have either jacked up the retail list price of drugs that have long been on the market after acquiring their patents, which is what Mylan did with EpiPen, or have imposed sky-high prices on newly developed, highly-effective drugs such as Gilead Sciences’ hepatitis-C drugs Sovaldi and Harvoni, which retail for roughly $1,000 per pill, or $84,000 for a course of treatment.
Expensive drugs have greatly added to the overall annual cost of U.S. health care and are posing serious economic consequences for consumers, health insurers and federal government agencies and programs.
Medicare Part D spending on EpiPen injectors, for example, grew by an astounding 1,151 percent between 2007 and 2014 – from $7 million to $87.9 million— while the number of beneficiaries rose by just 164 percent, according to a new study by the Kaiser Family Foundation released this week.
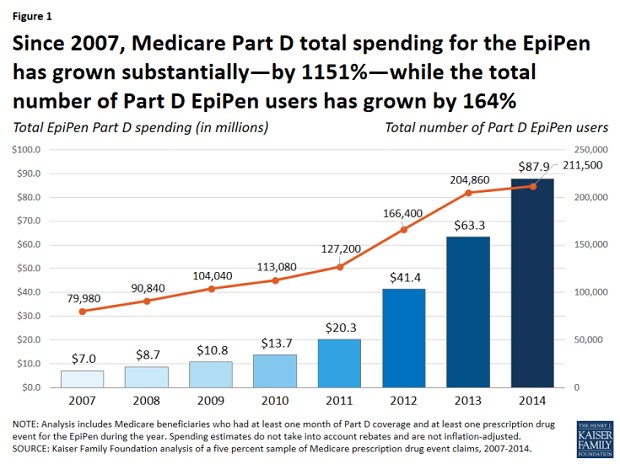
Moreover, out-of-pocket spending by seniors enrolled in Medicare Part D who didn’t qualify for income-based subsidies for EpiPen purchases nearly doubled during that period, from $30 to $56 per purchase. In the aggregate, out-of-pocket spending by all Part D enrollees who used the EpiPen increased more than five-fold between 2007 and 2014, according to the report, from $1.6 million to $8.5 million, “reflecting both an increase in the number of users and price increases for the EpiPen.” And that doesn’t include subsequent price hikes over the past two years that led to the current controversy.
Related: EpiPen Maker Enters the Pharmaceutical Industry’s Hall of Shame
Mylan has sought to quiet the uproar recently by offering consumers and insurers a new generic brand of the EpiPen for half the price – or $300 for a two-pack – which is scheduled to be brought out soon. Mylan also has agreed to bolster its consumer assistance program to cover the drug costs by doubling the income level at which families would still qualify for assistance.
Bresch emphasized in her written testimony that her company donated hundreds of thousands of EpiPen injectors to schools to help safeguard children from potentially fatal allergic reactions. She also noted that her company is engaged in research to increase the shelf life of the product, to save parents and schools money in the long term.
“I hope these facts will be considered in the larger discussion about the price,” she said in her testimony to the House Committee on Oversight and Government Reform. “Price and access exist in a balance, and we believe we have struck that balance.”
But Mylan’s latest actions are unlikely to satisfy lawmakers. “The EpiPen is a matter of life and death for some patients, and we have heard from far too many over the past several weeks about how patients are feeling forced to go without due to the increased cost,” members of the House Energy and Commerce subcommittee on oversight and investigations told Bresch in a recent letter.
Related: Two Big Reasons Prescription Drug Prices Are So Much Higher in the US
Earlier this month, New York state's attorney general Eric Schneiderman launched an investigation of Mylan with an eye to possible anti-competitive practices by the pharmaceutical giant in the sale of EpiPen to local school systems. Schneiderman's action Sept. 6 came within hours of Democratic Sens. Richard Blumenthal of Connecticut and Amy Klobuchar of Minnesota requesting that the Federal Trade Commission investigate whether Mylan violated federal antitrust laws to protect Epi-Pen from competition.
Bresch, the daughter of Democratic Sen. Joe Manchin III of West Virginia, worked her way up the ladder at Mylan and was named CEO in 2012. She was one of Fortune’s "50 Most Powerful Women In Business" in 2014. Last year, she received a total of $18.9 million in compensation.
According to a new USA Today investigation, the same year Bresch took the reins of the company, her mother, Gayle Manchin, helped “pave the way” for states to require public schools to keep EpiPen injectors in stock. Gayle Manchin spearheaded that drive after she became head of the National Association of State Boards of Education.
Thanks to that intervention, Mylan secured “a near monopoly in school nurses’ offices,” according to the news organization’s account. Eleven states drafted laws requiring epinephrine auto-injectors in the schools as a precaution. And nearly every other state subsequently followed suit after President Obama signed the 2013 School Access to Emergency Epinephrine Act, which gives funding preference to states that require EpiPen in their schools.